CONTEXT: Aplastic anemia (AA) is a rare disease with changing treatment modalities over time. Patients often do not respond to first line therapy and require multiple lines of therapy. Treatment has also evolved over time, with the addition of thrombopoietin receptor agonists. Understanding treatment response and outcomes remains imperative.
AIMS: To categorize treatments and outcomes of aplastic anemia patients
DESIGN: Single center retrospective study of 96 patients who were seen at Moffitt Cancer Center with a diagnosis of Aplastic Anemia between January 2009 and January 2023.
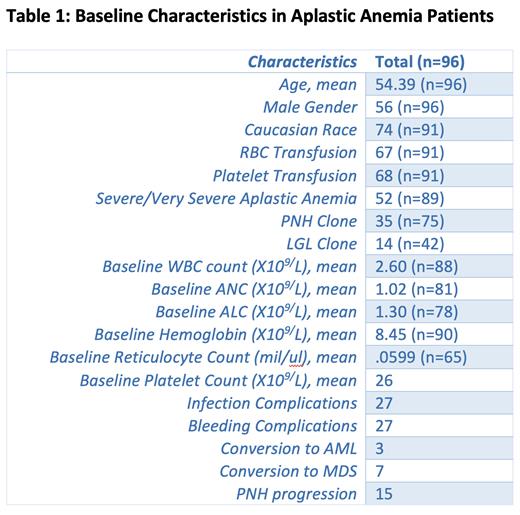
RESULTS: Our study included 96 patients (mean age at diagnosis, 54 years) diagnosed with AA. 56 patients (58%) were male, while 41 patients (42%) were female. Seventy-four (74%) were Caucasian, 7 (7%) were African American, 10 (10%) were Hispanic. At diagnosis, mean white blood count was 2.60, with an absolute neutrophil count of 1.02, a hemoglobin of 8.45, and platelet count of 26. At diagnosis, 67/91 (70%) patients had required packed red blood cell transfusion, while 68/91 (71%) had required platelet transfusion. In total, 77/91 (80%) required either a packed red blood cell or platelet transfusion at the time of diagnosis. At diagnosis, 27 patients had infectious complications while 27 also had bleeding complications.
Bone marrow cellularity was 10% or less in 59/86 patients (69%), and under 15% in 71 (83%). Forty-two patients were tested for large granular lymphocyte (LGL) clones, and 14 (33%) had clones present. Paroxysmal nocturnal hematuria (PNH) clones were detected in 35 out of the 75 patients tested (47%). The clones progressed in 14 patients. Seven patients had transformation from AA into Myelodysplastic syndrome (MDS), while 3 had transformation into Acute Myeloid Leukemia (AML).
Of the 96 patients, 6 patients were managed with observation. The most used frontline therapies were equine ATG (eATG) + cyclosporine (CSA) in 41 patients, followed by eATG + CSA + eltrombopag in 20 patients. In total, 65 patients received eATG based therapy first line (4 patients received eATG alone frontline). Eleven patients received an eltrombopag based therapy without concomitant ATG. Between singlet/doublet eATG based therapy and triplet therapy, the overall response rate to singlet/doublet was 66% (complete response rate (CR) 23%, partial response rate (PR) 43%) and 67% for triplet (CR 28%, PR 39%) (p=.91). There was no difference to date in OS. There was also no difference in response based on the presence on PNH clones or LGL clones. When looking specifically at severe/very severe AA, triplet CR was 30%, while singlet/doublet was 19%, showing a higher trend of CR in those patients receiving triplet therapy and severe/very severe AA. Thirty-eight patients who received eATG based singlet/doublet or triplet therapy upfront also received a second line treatment. The most common treatments were eltrombopag in 8 and Allogenic Hematopoietic Stem Cell Transplant (AHSCT) in 7. Fifteen had no response, 13 had a partial response, and 10 had complete response.
Three patients received AHSCT as a first line therapy, while another 19 patients received it second line or greater. AHSCT was second line for 10 patients, third line for 5, and fourth line for 3. Of these 22 patients, 2 had documented relapse (after receiving it as second line therapy) and were retransplanted. One developed MDS after AHSCT.
CONCLUSIONS: AA is diagnosed primarily in symptomatic patients requiring transfusions with life threatening cytopenias. Frontline treatment varies, but the most common therapies, which rely on eATG and CSA, as well as more recently thrombopoietin receptor agonists, have shown the most success. Our data showed that triplet therapy, incorporating eATG, CSA, and eltrombopag had a similar overall response rate, but that CR was higher in patients with severe/very severe AA receiving triplet versus singlet/doublet therapy upfront. Patients often require multiple lines of treatment. AHSCT remains a viable treatment option, with great response, but is not without its drawbacks.
Disclosures
Chan:BMS: Honoraria; AbbVie: Honoraria. Kuykendall:GSK: Consultancy; Imago: Consultancy; Prelude: Research Funding; Blueprint: Consultancy, Research Funding, Speakers Bureau; Morphosys: Consultancy, Research Funding; AbbVie: Consultancy; Protagonist Therapeutics, Inc.: Consultancy, Research Funding; CTI: Consultancy; Sierra Oncology: Research Funding; BMS: Consultancy, Research Funding; Incyte: Consultancy; Novartis: Consultancy. Padron:BMS: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees; Pharmaessentia: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Kura: Research Funding; CTI: Membership on an entity's Board of Directors or advisory committees; Gillead: Membership on an entity's Board of Directors or advisory committees. Sweet:Incyte: Research Funding; Nkarta: Consultancy; Jazz Pharmaceuticals: Consultancy, Research Funding; Daiichi Sankyo: Consultancy; Mablytics: Consultancy; Pfizer: Consultancy; Curis: Consultancy; Novartis: Consultancy; Arog: Consultancy; BerGenBio: Consultancy; Astellas: Consultancy; Bristol Myers Squibb: Consultancy; Gilead: Consultancy; BeiGene: Current Employment. Lancet:AbbVie Inc.: Consultancy; Atheneum: Consultancy; BerGenBio / DAVA Oncology: Consultancy; Boxer Capital: Consultancy; Celgene: Consultancy, Research Funding; Globe Life Sciences: Consultancy; Jasper Therapeutics: Consultancy; Jazz: Consultancy; MD Anderson: Consultancy; MEDTalks: Consultancy; Novartis: Consultancy; Peer Voice: Consultancy; Servier: Consultancy; Tegus: Consultancy; The Dedham Group: Consultancy. Sallman:AbbVie, Affimed Gmbh, Gilead, Incyte, Intellisphere, LLC, Molecular Partners AG, PGEN Therapeutics, Inc., Takeda, Zentalis; Advisory board for AvenCell, BlueBird Bio, BMS, Intellia, Jasper Therapeutics, Kite, Magenta Therapeutics, NKARTA, Novartis, Orbita: Consultancy; Aprea, Jazz: Research Funding. Jaglal:Janssen: Membership on an entity's Board of Directors or advisory committees; SOBI: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees. Komrokji:Geron: Consultancy; Rigel, Taiho, DSI: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie, CTI biopharma, Jazz, Pharma Essentia, Servio: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal